Chemistry Form 2 Topic 6
PERIODIC CLASSIFICATION
Constructing the modern periodic table has been a major scientific achievement. The first steps towards working out this table were taken long before anyone had any idea about the structure of atoms. The number of elements discovered increased steadily during the 19th century. Chemists began to find out patterns in their properties.
The Law of Triads
In 1817, the German scientist Johann Dobereiner noticed that calcium, strontium and barium had similar properties, and that the atomic weight of strontium was halfway between the other two. He found the same pattern with chlorine, bromine and iodine and also with lithium, sodium and potassium. So, he put forward the law of Triads: “If elements are arranged in groups of three in order of increasing atomic weights, having similar properties, then the atomic weight of the middle element is the arithmetic mean of the atomic weights of the other two elements”, E.g.
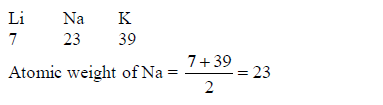
The following are examples of Dobereiner’s triads:(Lithium, Sodium and Potassium)(Calcium, Strontium and Barium)(Chlorine, Bromine and Iodine) and(Iron, Cobalt and Nickel)
The Law of Octaves
In 1863 John Newlands, an English chemist noted that there were many pairs of similar elements. In each pair, the atomic weights differed by a multiple of 8. So, he produced a table with the elements in order of increasing atomic weights, and put forward the Law of Octaves: “If elements are arranged in order of their increasing atomic weights, the properties of the 8th element, starting from a given one, are a kind of repetition of the first element”.
This finding was comparable to the 8th note of music, hence the use of the word “octave”.
This was the first table to show a periodic or repeating pattern of properties. But it was not widely accepted because there were too many inconsistencies. For example, he put copper and sodium in the same group, even though have very different properties. Also iron was placed in the same group as oxygen and sulphur.
The Periodic Law
Dmitri Mendeleev was born in Siberia, Russia, in 1834. By the time he was 32, he was a professor of Chemistry. In 1869 Mendeleev advanced the work done by Newlands and contributed very useful new ideas. He began by listing all the known elements in order of increasing atomic mass. He spotted that elements with similar properties appear at regular intervals or periods down the list. His findings were the basis for the Periodic Law: “The properties of elements are a periodic function of their atomic masses”.
Mendeleev placed similar elements into groups. He realized that not all elements had been discovered. So he left gaps for new ones in the correct places in his table. He also swapped the order of some elements to make them fit better. He predicted the properties of the missing elements from the properties of the elements above and below them in the table. He also listed separately some elements which did not appear to fit into any group i.e. iron, cobalt, nickel, etc.
Table 6.1: Mendeleev’s short form of the Periodic Table
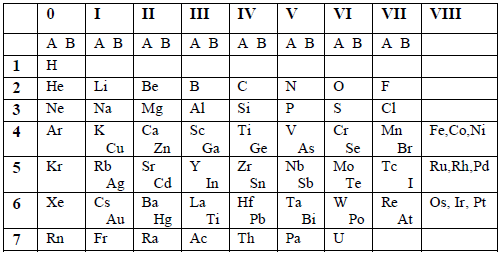
The table had 9 vertical columns which he called Groups. The groups were numbered from 0 to 8. The elements in group 0 were not known by then, but were discovered later on. Groups 1 to 7 were subdivided into A and B subgroups. Group 0 included the transition elements. Noble gases were later placed in group 0.
There were 7 horizontal rows which he called periods. All vacant positions in the table stood for new elements yet to be discovered.
Usefulness of Mendeleev’s classification
- The table summarized a large amount of information about the elements based on their chemical properties.
- The table was very useful in predicting the existence and properties of undiscovered elements, for which gaps had been left in the table.
- The table was also used in checking relative atomic masses of elements.
Limitations of Mendeleev’s classification
- In three cases, pairs of elements had to be included in one group based on inverse order of their atomic weights so as to fit into groups of elements having similar properties. These pairs were argon (39.9) and potassium (39.1), cobalt (58.9) and nickel (58.9); plus tellurium (127.5) and iodine (126.9). This difficulty was resolved when the basis of classification was based on the atomic number instead of the atomic mass.
- The elements that were placed in group VIII formed an incompatible mixture.
- The placing of two different families in one group e.g. K and Cu; Ca and Zn, etc.
The periodic table is the chemists map. It helps you understand the patterns in chemistry. Today we take it for granted. But it took hundreds of years, and work of hundreds of chemists, to develop.
The Modern Periodic Table is similar to that of Mendeleev, but contains several improvements. Elements are arranged in order of atomic number instead of atomic mass. This means that elements no longer have to swap places to fit correctly. Many new elements have been discovered and slotted into the spaces left by Mendeleev. Also metals and non-metals are clearly separated. The Modern Periodic Table is shown in Figure 6.1.
Figure 6.1: The Modern Periodic Table
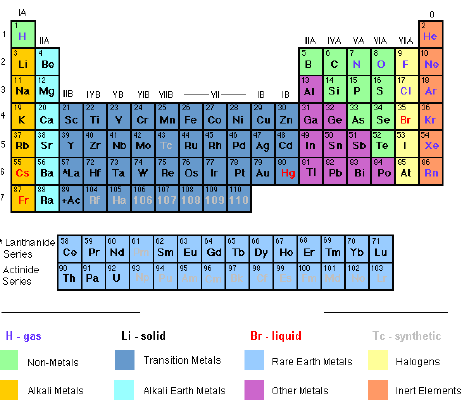
The long form of the periodic table is the commonly used form of the periodic table. The elements in the table are arranged based on their atomic weights, starting from hydrogen (1), helium (2), lithium (3), beryllium (4) and so on. The elements appear in vertical columns and horizontal rows.
The vertical columns in the table are called Groups, numbered I, II, III, IV, V, VI, VII and 0, which is also known as group VIII. Group I contains the elements lithium (L), sodium (Na), rubidium (Rb), caesium (Cs) and francium (Fr). Group II consists of elements starting from sodium (Na) down to radium (Ra). Some of the groups have special names.
- Group I is often called the alkali metals.
- Group II the alkaline earth metals.
- Group VII the halogens.
- Group 0 the noble gases.
The transition metals (or elements) form a separate block in the middle of the periodic table between group II and III. The atoms of these elements have more complicated electron arrangements. Note that the group contains many common metals such as iron (Fe), Nickel (Ni), copper (Cu), and Zinc (Zn). One of the interesting properties of these elements is that they form coloured compounds.
Main features of the Modern Periodic Table
- The elements in the table are placed in order of their atomic numbers instead of their atomic masses.
- There are a total of 18 groups and 7 periods.
- There are 5 blocks of similar elements in the periodic table as shown in figure 6.2.
- The normal (non-transition) elements (groups 1-7) have their outermost shells incomplete, meaning that they can allow additional electrons to enter into their outermost orbital (valency shell). But each of their inner shells is complete.
- The transition metals have their outermost as well as their penultimate (second last) shells incomplete.
- Elements of group 0 (noble gases) have their shells complete. These elements show little reactivity. That is why they wereonce called „inert‟ gases because they are very unreactive; or „rare gases‟ because they were rarely found.
- Gaps left by Mendeleev for undiscovered elements (now occupied by the transition elements and the noble gases) have been filled by the respective elements following their discovery. Man-made elements have also found a place in the periodic table.
- Metals have been clearly separated from non-metals. Metalloids or semi metals (poor metals) have also been included. Metalloids are elements whose properties are intermediate between metals and non-metals. They include boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb) and tellurium (Te). In some publications, germanium and antimony are usually classed as poor metals and the rest as non-metals.

Periodicity
The Concept of Periodicity
Explain the concept of periodicity
Consider the electronic configuration of the first twenty elements of the periodic table shown in the table below.
Table 6.3: Electronic configurations of the first 20 elements
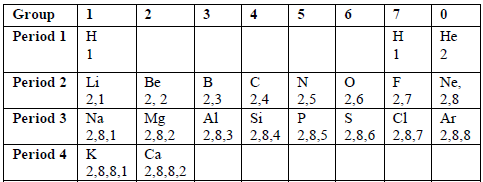
You will notice that elements in the same vertical columns (groups) have the same number of electrons in the outermost shells of their atoms. Because the outer electrons determine the chemical properties of an element, then the elements in each period tend to resemble each other closely in chemical behaviour. For instance, the noble gases, He, Ne and Ar show a chemical inertness which is characterised by the stable outer electron octet or duplet. Due to this reason, the compounds of the noble gases with other elements have not been found.
Attempts to classify elements by arranging them in order of increasing atomic weights shows that the properties of elements were periodic. This means elements with similar or comparable properties appear after a certain specific interval in a given arrangement. The occurrence of successive groups of elements showing strong chemical similarity in this way is called periodicity.
Therefore, periodicity is the repetition of similar chemical properties of elements after a certain specific interval in a given arrangement. The repetition in properties is due to repetition of similar electronic configuration of outermost shells of elements after certain intervals.
General Trends
This refers to change in some properties of elements across the periods and down the groups in the periodic table. These trends become more obvious if we leave aside the noble gases in Group 0. In this case, we shall concentrate our efforts on variations in the most important properties of the elements only. The following is a summary of the change in some properties of elements down the groups and across the periods.
The Change in Properties of Elements Across the Periods
Explain the change in properties of elements across the periods
Atomic and ionic size
The sizes of atoms and ions may be given in terms of atomic radius and ionic radius units respectively. The number of shells an atom or ion posses and the nuclear charge determines the size of an atom or ion. This is how the two properties vary along the period and down the group:
Atomic size
Along the period: Considering the normal elements only, the size of the atoms decrease from left to right across the period. This is because as atomic number increases across the period, the nuclear charge (due to increasing protons) increases and electrons in shells are pulled closer to the nucleus.
Ionic size
- Positive ions (cations):Across the period; The ionic size does not change, i.e. remains the same, as you move across the period from either direction.
- Negative ions (anions):A negative ion is larger compared to the corresponding neutral atom because on forming an ion, one or more electrons are added to the atom. The added electron(s) is/are repelled by the electron(s) already present in the outermost shell, hence leading to an increase in the size of an atom, even though no new shell is formed.Down the group and along the period: Ionic size increases down the group, and along the period, i.e. from left to right.
Atomic radii (singular: radius)
Along the period: In the period, atomic radii decrease from left to right with increase in the atomic number.
Electronegativity
Electronegativity is the tendency of an atom to attract the shared pair of electrons towards itself in a molecule. The electronegativity values of elements in group 0 (inert gases) is zero.
Along the period: Electronegativity increases while moving across the period from left to right in the periodic table.
Metallic character (or electropositivity)
Electropositivity is the tendency of an element to lose the valency electron(s) and donate the same to other elements (usually non-metallic elements). This process occurs during the formation of new substances e.g. molecules and compounds. Literally, such reactions occur between metals and non-metals whereby metals donate electrons and non-metals receive these electrons. So, metals are electropositive elements while non- metals are electronegative elements.
Along the period: Generally, metallic character decreases along the period from left to right.The gradation in metallic properties across the period is as follows: Metals → poor metals → metalloids → non-metals → noble gases
Chemical reactivity
Reactivity is the tendency of an element to lose or gain electrons in a chemical reaction.
Along the period: For metals, the reactivity decreases from left to right in a period while it increases for non-metals.
Ionization Energy or Ionization Potential (I.E or I.P)
This refers to the minimum amount of energy required to remove the most loosely bound electron from an isolated atom or ion in its gaseous state. The smaller the value of ionization energy, the easier it is to remove the electron from the atom.M(g) →M+(g) + e-
Along the period: It increases along the period from left to right with the increase in atomic number.
Electron affinity (Ea):
This is just opposite to I.E. It is defined as the amount of energy released when an extra electron is added to an isolated neutral atom in its gaseous state.
Along the period: The value increases along the period from left to right.
Density and melting point
The density of a substance is the ratio of its mass to its volume, while the melting point is the temperature at which a solid substance turns into liquid at standard atmospheric pressure.
- Density-Across the period: Densities decrease across the period from left to right.
- Meting point-Across the period: Melting points of elements decrease across the period from left to right.
The Change in Properties of Elements Down the Groups
Explain the change in properties of elements down the group
Atomic and ionic size
- Atomic size-Down the group: Atomic size increases as you move down the group.
- Ionic size- Positive ions (cations)-Down the group: On descending the group, the nuclear charge increases and the number of shells increase by one at each step so, the ionic size also increases. A positive ion is smaller than the corresponding neutral atom because on forming the ion, the metal atom loses both the valency electron(s) and the outermost shell. Valency electron(s) refer(s) to the electron(s) in the outer-most shell of an atom. Any further removal of electron(s) from the ion will decrease the ionic size further.Negative ions (anions)-Down the group and along the period: Ionic size increases down the group, and along the period, i.e. from left to right.
Atomic radii (singular: radius)
Atomic radius is the distance from the centre of the nucleus to the outermost shell (valency shell). Down the group: Atomic radii of elements increase down the group with increase in atomic size.
Electronegativity
Down the group: Electronegativity decreases while moving downwards in a group.
Metallic character (or electropositivity)
Down the group: Metallic character (electropositivity) increases down the group
Ionization Energy or Ionization Potential (I.E or I.P)
Down the group: It decreases gradually down the group.
Why is there a decrease in I.E as you go down the group? This is because electrons are held in their shells by their attraction to the positive nucleus, and as you go down the group, the size of the atom increases (increasing atomic radius). So, the outermost electron(s) of an atom gets further and further away from the attraction or pull of the positive nucleus, hence requiring little energy to remove from the atom.
Electron affinity (Ea)
Down the group: The value of electron affinity decreases down the group.
Density and melting point
- Density-Down the group: Densities of elements increase down the group.
- Meting point-Down the group: Melting points of elements decrease down the group as the elements become less metallic in nature.
Electronic Configuration to Locate the Positions of Elements in Periodic Table
Use electronic configuration to locate the positions of elements in periodic table
The modern periodic table is based on electronic configurations of the elements. Look at table 6.3 and study the electronic configurations of the first twenty elements and where they are placed in the periodic table.
Beryllium, magnesium and calcium have two electrons in the outer shell. These elements are in Group 2.
This pattern continues to Group 3, Group 4 and so on. The group number in the periodic table is the same as the number of electrons in the outermost shell. The halogens are the elements in Group 7. Bromine is one of the halogens. How many electrons does each bromine atom have in its outer shell?
As we move down each group, the number of shells increases by one at each step. Each atom of an element has one complete shell than the one above it.
As we move across each period, the outer shell is being filled by one electron at each step. Certain electronic configurations are found to be more stable than others are. The noble gases at the end of each period have full outer shells. They have stable duplet (2 electrons) or octet (8 electrons) in their outermost shells. This makes them more difficult to break up, and this fits well with the fact that they are so unreactive.
The outer electrons of an atom are mainly responsible for the chemical properties of an element. Therefore, elements in the same group will have similar chemical properties.
- READ TOPIC 7: Formula Bonding and Nomenclature